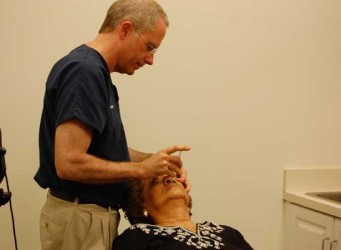
A new device developed by Retina Health Center to improve eye injections is featured in the January edition of Review of Ophthalmology, a medical publication which highlights current, clinically relevant information on surgical techniques, disease diagnosis and management and new technologies in the field of ophthalmology.
Recently developed by Retina Health Center in collaboration with other researchers, the guarded injection device incorporates a small gauge needle covered by a thin protective sleeve designed to protect the needle from contamination risks before and during the injection such as aerosolized saliva droplets from speech or breathing, as well as from the eyelashes or other external contaminants. Clinical trials of the investigational device recently began at the Retina Health Center to evaluate the comfort and effectiveness of the device.
In the article titled, “Striving to Improve Intravitreal Injections, Eaton says he anticipates that the guarded injection device will be available in the marketplace within six months to a year. Click here to read the full article: http://www.revophth.com/content/d/technology_update/i/2251/c/38568/.
This is the first of a number of products being developed by I-Tech JV Development Company which address issues with intravitreal injections to reach clinical trial. By combining a team of retinal thought leaders and experienced device designers, the I-Tech JV Development Company is able to rapidly assess and develop products to meet evolving retina needs.
Retina Health Center and the Macular Degeneration Research Center were established in 2002 by Dr. Alexander M. Eaton, a long-time Southwest Florida resident who has been practicing ophthalmology in Lee and Collier counties for more than 17 years. Dr. Eaton has been the principal investigator for numerous studies to prevent and treat macular degeneration. For more information on the latest studies or to make an appointment, call 239-337-3337 in Fort Myers or 239-793-5200 in Naples.